We return with another new installment of advances against cancer! This week we will talk about cellular senescence, a key mechanism in our cells to prevent the development of cancer. In turn, we will analyze a second generation drug aimed at inducing the death of senescent cancer cells that accumulate as a result of chemotherapy … KEEP READING!
Cancer is a disease that originates from the accumulation of several mutations in the genome of a single cell. This cell begins to multiply in an uncontrolled way and then the tumor appears, an accumulation of malignant cells.
However, our cells have certain security mechanisms to deal with this accumulation of mutations. On the one hand, DNA repair systems that try to correct these alterations before they become irreversible and are already considered mutations.
On the other hand we have the so-called tumor suppressors that if they detect errors in DNA, when a cell wants to divide they act and prevent it. What is the use of transmitting mutations to new cells when there are so many others healthy and ready to multiply? Furthermore, cells are capable of inducing their own death when they see that it is the best solution so as not to endanger the entire organism. This process is called apoptosis.
On the other hand, we must mention telomerase. Telomerase is the enzyme that keeps telomeres long, repetitive DNA sequences that are located at the ends of our chromosomes. These telomeres are in charge of allowing the chromosomes to be copied in each cell division in order to guarantee that the two new resulting cells acquire the entire genomic endowment. The problem is that in each division those telomeres are shortened.
When these telomeres are too short, the cell is unable to copy its genetic material and therefore to divide. The vast majority of our cells have their telomerase inactive. If we think about it, it is another mechanism that prevents the cell from multiplying indefinitely, limiting the potential development of a cancer.
On the other hand we have protoncogenes, genes that, unlike suppressors, are going to favor the development of cancer. If these genes are altered by a mutation, they become over-activated (oncogenes), exaggerating their function: proliferation, cell division or the arrest of cell death. For this reason it is said that they “go for” cancer.
Therefore, both mutations in protoncogenes, as in suppressors, as in telomerase or DNA repair genes are related to the development of cancer.
But why are we talking about all this? Because senescence is the ability of cells to stop their own growth. And the reasons they prefer to stop growing than to continue proliferating are the ones we just mentioned. First, when they detect that the telomeres are already too short and the chromosomes cannot be copied (it is called replicative senescence). Additionally, when “possible tumor” alarms go on. In the latter case, from the accumulation of mutations in key genes in tumor development (oncogenes and tumor suppressors), to the repair systems that precisely correct and mutates cannot, as well as some structural changes of the chromosomes.
In these situations the cell enters senescence, a state in which it remains without growing or proliferating. This senescence is precisely related to aging. Let’s take a very simple example to understand it: if the cells that make up the skin of your face go into senescence they will not renew, so they will continue to deteriorate and the signs of aging will appear: wrinkles, blemishes … That is, the cells they are not renewed, they accumulate by aging.
As we can see, senescence, although related to aging, is in turn a protective system against cancer. And in fact, it is the goal of therapies like chemotherapy. Chemotherapy drugs aim to damage the DNA of dividing tumor cells. Thus, the response of these tumor cells is to enter senescence. Ironically, it has been observed that the accumulation of these tumor senescent cells can have a counterproductive effect, since in the end they acquire the ability to reverse that senescence process with an even higher tumorigenic potential.
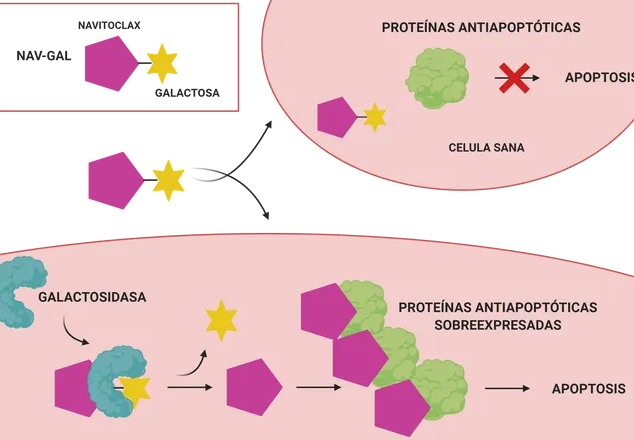
For all these reasons, we can explain the search for therapies that permanently eliminate these senescent cells from the tumor to avoid reversals of the process. These drugs (called senolytics) target precisely these senescent tumor cells. However, not everything is good news since they have shown a high mainly hematological toxicity, causing even
This new drug has been successfully tested in animal models of lung cancer, in which combined with chemotherapy (cisplatin) slow tumor growth. Furthermore, it has been tested, in human (as well as animal) blood, that the compound does not cause the toxicity of first-generation senolytics on platelets.
And the question that would come to me at this point is the following: How can a galactose molecule give specificity for senescent cells to a drug? First, the authors confirm that the drug enters both senescent and non-senescent cells. The question kit lies in that only senescent ones have galactosidase activity. Having galactosidase activity comes to mean that the cell contains galatosidase enzymes, proteins that are responsible for breaking down this sugar.
Only senescent cells will therefore be able to break down the sugar that the drug has combined. This sugar-bound drug is unable to interact with its target proteins. But once the sugar is removed, you can join them. This molecule inhibits the BCL-2, BCL-X (L) and BCL-W proteins, responsible for slowing down the death of a cell (and over-expressed in a tumor). Thus, the drug has the ability to induce apoptosis of said cell.
Let’s recap to make sure we’ve got all the ideas. Senescence is a process that occurs in cells that accumulate errors in their DNA, as well as in cells that do not have telomeres long enough to continue multiplying. In response to this situation, the cell enters senescence, a state in which it remains without multiplying, aging (in the end it continues to accumulate problems and is not renewed).
Many chemotherapeutic drugs induce, precisely, the senescence of tumor cells, since they damage their DNA and stop their proliferation. The problem is that this state can be reversed and cancer cells can acquire even greater malignant potential. Thus, drugs such as Navitoclax have been developed, which induce apoptosis (death) of senescent cells, but have too much toxicity in healthy cells.
This group of researchers has managed to reduce this toxicity, conjugating the drug Navitoclax with a sugar. In this way, the drug only acts on cells that have galactosidase activity (senescent tumors). The result? Its apoptosis and, in combination with chemotherapy, the reduction of tumor size.
For the moment? Wait for investigations to progress. The success of this strategy in cell cultures (in vitro) and in animals (in vivo) does not necessarily imply that the therapy is effective, and safe, also in humans. Therefore, before its approval, it must be tested in clinical trials, already in patients.