Nattrols are inactivated controls for Diagnostic tests by real time PCR orGenExpet, Cepheid.
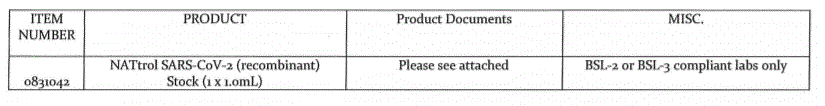
Lieven@gentaur.com for more information about nucleic acid controls.
We can deliver next day to all offical government labs in Europe in April 2020. We need your BSL-2 certification before ordering.
|
NATSARS-ST NATtrol Coronavirus SARS Stock |
NATMERS-ST NATtrol MERS-CoV Stock |
OTHER PCR Controls
Molecular Biology – Qualitative Standards-Controls
|
NATCOV(229E)-ST NATtrol Coronavirus 229E |
NATCOV(NL63)-ST NATtrol Coronavirus NL63 |
NATCOV(OC43)-ST NATtrol Coronavirus OC43 |
Organisms – Culture Fluids Heat Inactivated
|
0810024CFHI Coronavirus Strain: OC43 – CFHI |
0810228CFHI Coronavirus Strain: NL63 – CFHI |
0810229CFHI Coronavirus Strain: 229E – CFHI |
Organisms – Purified Viral Lysate Heat Inactivated
|
0810024 Coronavirus OC43 Lysate |
0810228 Coronavirus NL63 Lysate |
0810229 Coronavirus 229E Lysate |
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study.
Since December, 2019, Wuhan, China, has experienced an outbreak of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Epidemiological and clinical characteristics of patients with COVID-19 have been reported but risk factors for mortality and a detailed clinical course of illness, including viral shedding, have not been well described.In this retrospective, multicentre cohort study, we included all adult inpatients (≥18 years old) with laboratory-confirmed COVID-19 from Jinyintan Hospital and Wuhan Pulmonary Hospital (Wuhan, China) who had been discharged or had died by Jan 31, 2020. Demographic, clinical, treatment, and laboratory data, including serial samples for viral RNA detection, were extracted from electronic medical records and compared between survivors and non-survivors. We used univariable and multivariable logistic regression methods to explore the risk factors associated with in-hospital death.191 patients (135 from Jinyintan Hospital and 56 from Wuhan Pulmonary Hospital) were included in this study, of whom 137 were discharged and 54 died in hospital. 91 (48%) patients had a comorbidity, with hypertension being the most common (58 [30%] patients), followed by diabetes (36 [19%] patients) and coronary heart disease (15 [8%] patients). Multivariable regression showed increasing odds of in-hospital death associated with older age (odds ratio 1·10, 95% CI 1·03-1·17, per year increase; p=0·0043), higher Sequential Organ Failure Assessment (SOFA) score (5·65, 2·61-12·23; p<0·0001), and d-dimer greater than 1 μg/L (18·42, 2·64-128·55; p=0·0033) on admission. Median duration of viral shedding was 20·0 days (IQR 17·0-24·0) in survivors, but SARS-CoV-2 was detectable until death in non-survivors. The longest observed duration of viral shedding in survivors was 37 days.
The potential risk factors of older age, high SOFA score, and d-dimer greater than 1 μg/L could help clinicians to identify patients with poor prognosis at an early stage. Prolonged viral shedding provides the rationale for a strategy of isolation of infected patients and optimal antiviral interventions in the future.
Source: Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences; National Science Grant for Distinguished Young Scholars; National Key Research and Development Program of China; The Beijing Science and Technology Project; and Major Projects of National Science and Technology on New Drug Creation and Development.
Early dynamics of transmission and control of COVID-19: a mathematical modelling study.
An outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to 95 333 confirmed cases as of March 5, 2020. Understanding the early transmission dynamics of the infection and evaluating the effectiveness of control measures is crucial for assessing the potential for sustained transmission to occur in new areas. Combining a mathematical model of severe SARS-CoV-2 transmission with four datasets from within and outside Wuhan, we estimated how transmission in Wuhan varied between December, 2019, and February, 2020. We used these estimates to assess the potential for sustained human-to-human transmission to occur in locations outside Wuhan if cases were introduced.We combined a stochastic transmission model with data on cases of coronavirus disease 2019 (COVID-19) in Wuhan and international cases that originated in Wuhan to estimate how transmission had varied over time during January, 2020, and February, 2020. Based on these estimates, we then calculated the probability that newly introduced cases might generate outbreaks in other areas.
To estimate the early dynamics of transmission in Wuhan, we fitted a stochastic transmission dynamic model to multiple publicly available datasets on cases in Wuhan and internationally exported cases from Wuhan. The four datasets we fitted to were: daily number of new internationally exported cases (or lack thereof), by date of onset, as of Jan 26, 2020; daily number of new cases in Wuhan with no market exposure, by date of onset, between Dec 1, 2019, and Jan 1, 2020; daily number of new cases in China, by date of onset, between Dec 29, 2019, and Jan 23, 2020; and proportion of infected passengers on evacuation flights between Jan 29, 2020, and Feb 4, 2020. We used an additional two datasets for comparison with model outputs: daily number of new exported cases from Wuhan (or lack thereof) in countries with high connectivity to Wuhan (ie, top 20 most at-risk countries), by date of confirmation, as of Feb 10, 2020; and data on new confirmed cases reported in Wuhan between Jan 16, 2020, and Feb 11, 2020.
FINDINGS
We estimated that the median daily reproduction number (Rt) in Wuhan declined from 2·35 (95% CI 1·15-4·77) 1 week before travel restrictions were introduced on Jan 23, 2020, to 1·05 (0·41-2·39) 1 week after. Based on our estimates of Rt, assuming SARS-like variation, we calculated that in locations with similar transmission potential to Wuhan in early January, once there are at least four independently introduced cases, there is a more than 50% chance the infection will establish within that population.
Our results show that COVID-19 transmission probably declined in Wuhan during late January, 2020, coinciding with the introduction of travel control measures. As more cases arrive in international locations with similar transmission potential to Wuhan before these control measures, it is likely many chains of transmission will fail to establish initially, but might lead to new outbreaks eventually.Wellcome Trust, Health Data Research UK, Bill & Melinda Gates Foundation, and National Institute for Health Research.
KULeuven research group
Coronavirus Disease 2019 (COVID-19): What we know?
In late December 2019, a cluster of unexplained pneumonia cases has been reported in Wuhan, China. A few days later, the causative agent of this mysterious pneumonia was identified as a novel coronavirus. This causative virus has been temporarily named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the relevant infected disease has been named as coronavirus disease 2019 (COVID-19) by the World Health Organization respectively. The COVID-19 epidemic is spreading in China and all over the world now. The purpose of this review is primarily to review the pathogen, clinical features, diagnosis, and treatment of COVID-19, but also to comment briefly on the epidemiology and pathology based on the current evidences. This article is protected by copyright. All rights reserved.
Liver injury during highly pathogenic human coronavirus infections.
The severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), the pathogen of 2019 novel coronavirus disease (COVID-19), has posed a serious threat to global public health. The WHO has declared the outbreak of SARS-CoV-2 infection an international public health emergency. Lung lesions have been considered as the major damage caused by SARS-CoV-2 infection. However, liver injury has also been reported to occur during the course of the disease in severe cases. Similarly, previous studies have shown that liver damage was common in the patients infected by the other two highly pathogenic coronavirus – severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV), and associated with the severity of diseases. In this review, the characteristics and mechanism of liver injury caused by SARS-CoV, MERS-CoV, as well as SARS-CoV-2 infection were summarized, which may provide help for further studies on the liver injury of COVID-19.
Consensus of Chinese experts on protection of skin and mucous membrane barrier for healthcare workers fighting against coronavirus disease 2019.
Health professions preventing and controlling Coronavirus Disease 2019 are prone to skin and mucous membrane injury, which may cause acute and chronic dermatitis, secondary infection and aggravation of underlying skin diseases. This is a consensus of Chinese experts on protective measures and advice on hand-cleaning- and medical-glove-related hand protection, mask- and goggles-related face protection, UV-related protection, eye protection, nasal and oral mucosa protection, outer ear and hair protection. It is necessary to strictly follow standards of wearing protective equipment and specification of sterilizing and cleaning. Insufficient and excessive protection will have adverse effects on the skin and mucous membrane barrier. At the same time, using moisturizing products is highly recommended to achieve better protection. This article is protected by copyright. All rights reserved.
Impact of international travel and border control measures on the global spread of the novel 2019 coronavirus outbreak.
The novel coronavirus outbreak (COVID-19) in mainland China has rapidly spread across the globe. Within 2 mo since the outbreak was first reported on December 31, 2019, a total of 566 Severe Acute Respiratory Syndrome (SARS CoV-2) cases have been confirmed in 26 other countries. Travel restrictions and border control measures have been enforced in China and other countries to limit the spread of the outbreak.
We estimate the impact of these control measures and investigate the role of the airport travel network on the global spread of the COVID-19 outbreak. Our results show that the daily risk of exporting at least a single SARS CoV-2 case from mainland China via international travel exceeded 95% on January 13, 2020. We found that 779 cases (95% CI: 632 to 967) would have been exported by February 15, 2020 without any border or travel restrictions and that the travel lockdowns enforced by the Chinese government averted 70.5% (95% CI: 68.8 to 72.0%) of these cases. In addition, during the first three and a half weeks of implementation, the travel restrictions decreased the daily rate of exportation by 81.3% (95% CI: 80.5 to 82.1%), on average. At this early stage of the epidemic, reduction in the rate of exportation could delay the importation of cases into cities unaffected by the COVID-19 outbreak, buying time to coordinate an appropriate public health response.
Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients.
Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection.
It has been known that, the novel Coronavirus, 2019-nCoV, which is considered similar to SARS-CoV and originated from Wuhan (China), invades human cells via the receptor angiotensin converting enzyme II (ACE2). Moreover, lung cells that have ACE2 expression may be the main target cells during 2019-nCoV infection. However, some patients also exhibit non-respiratory symptoms, such as kidney failure, implying that 2019-nCoV could also invade other organs.
To construct a risk map of different human organs, we analyzed the single-cell RNA sequencing (scRNA-seq) datasets derived from major human physiological systems, including the respiratory, cardiovascular, digestive, and urinary systems. Through scRNA-seq data analyses, we identified the organs at risk, such as lung, heart, esophagus, kidney, bladder, and ileum, and located specific cell types (i.e., type II alveolar cells (AT2), myocardial cells, proximal tubule cells of the kidney, ileum and esophagus epithelial cells, and bladder urothelial cells), which are vulnerable to 2019-nCoV infection. Based on the findings, we constructed a risk map indicating the vulnerability of different organs to 2019-nCoV infection. This study may provide potential clues for further investigation of the pathogenesis and route of 2019-nCoV infection.
Genetic evolution analysis of 2019 novel coronavirus and coronavirus from other species.
The Corona Virus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a Public Health Emergency of International Concern.
However, so far, there are still controversies about the source of the virus and its intermediate host. Here, we found the novel coronavirus was closely related to coronaviruses derived from five wild animals, including Paguma larvata, Paradoxurus hermaphroditus, Civet, Aselliscus stoliczkanus and Rhinolophus sinicus, and was in the same branch of the phylogenetic tree.
Lieven Gevaert, Bio-engineer Gentaur Institute